
January 16, 2023 Puzzle Piece
Dr
Harris is administering MSC’s, Exosomes and D5W injections in
conjunction with Homecoming 2023. Call Jason to schedule at
480-964-5198.
Umbilical Cord Tissue-Derived Mesenchymal Stem Cells (UC-MSCs)
UC-MSCs can be sourced from a variety of areas including Wharton’s
Jelly, cord lining, and peri-vascular region of the umbilical cord. As a
commonly discarded tissue, the umbilical cord contains a rich source of
mesenchymal stromal cells, which are therefore obtained non-invasively
(14).
"UC-MSCs are the most primitive type of MSCs, shown by their higher expression of Oct4, Nanog, Sox2, and KLF4 markers." (1)
Umbilical cord tissue-derived mesenchymal stem cells have the ability to
differentiate into different cell types and have the greatest
proliferation rate of the three mentioned types of stem cells (adipose,
bone marrow, cord tissue). (2)
Similar to adipose tissue and bone marrow-derived MSCs, UC-MSCs are
known to secrete growth factors, cytokines, and chemokines, improving
different cell repair mechanisms. (4). These functions all assist the
anti-inflammatory and immunomodulatory properties of MSCs.
Non-invasive cell product
The harvesting procedure of UC-MSCs is non-invasive as it does not
require extraction from the patient. The MSCs are taken directly from
an area of an ethically donated human umbilical cord.
UC-MSCs also have a high proliferative potential than BMSCs and ASCs
meaning they expand in vitro more effectively allowing for greater
efficiency when obtaining higher cell numbers. (15)
Studies have found that UC-MSCs genes related to cell proliferation (
EGF), PI3K-NFkB signaling pathway (
TEK), and neurogenesis (
RTN1,
NPPB, and
NRP2) were upregulated (increase in the number of receptors) in UC-MSCs compared to in BM-MSCs. (15)
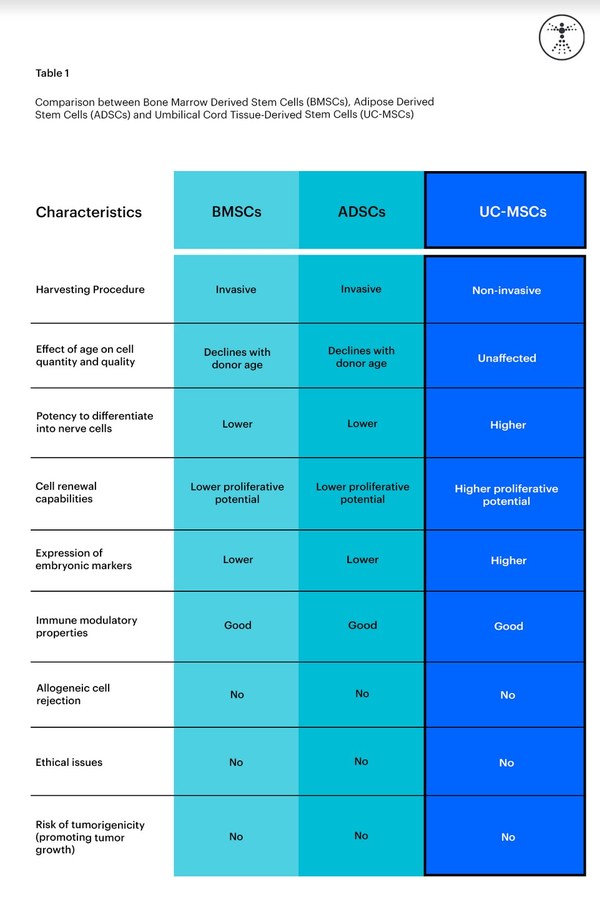
The image below shows a comparison between BMSCs, ADSCs, and UC-MSCs.
How do mesenchymal stem cells work in the body?
Mesenchymal stem cells utilize their self-renewal, immunomodulatory,
anti-inflammatory, signaling, and differentiation properties to
influence positive change within the body. Mesenchymal stem cells
(MSCs) also have the capacity to self-renew by dividing and developing
into multiple specialized cell types present in a specific tissue or
organ. Mesenchymal stem cells are adult stem cells, meaning they
present no ethical concerns, MSCs are not sourced from embryonic
material.
"The characteristics of presenting no major ethical concerns, having low
immunogenicity, and possessing immune modulation functions make MSCs
promising candidates for stem cell therapies." - Jiang, et al. (6)
Immunomodulatory (regulating the immune system)
Mesenchymal stem cells (MSCs) can regulate the immune system by
promoting an inflammatory response when the immune system is
under-activated and reducing inflammation when the immune system is
overactivated. MSCs can play a key role in preventing the immune system
from attacking itself similar to what one may see in many autoimmune
disorders. According to a 2013 study conducted by Bernardo et al. MSCs,
when exposed to sufficient levels of pro-inflammatory markers
(cytokines) respond by promoting an immune-suppressive response to
dampen inflammation and promote tissue homeostasis. (7)
According to a 2019 study conducted by Jiang, et al.
"Depending on the signal types or strength, MSCs secrete cytokines to
promote or suppress the immune responses for maintaining the immune
balance."
This balance is outlined by the same study in the figure below.
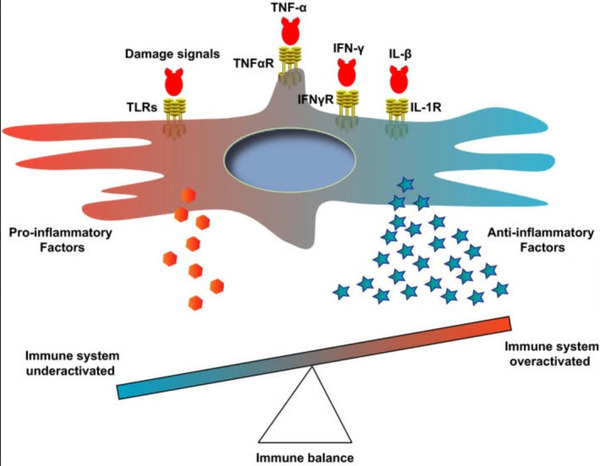
Figure 2. Table showcasing immune system balance
Anti-inflammatory (reducing harmful inflammation)
Inflammation is a response from the immune system that is aimed at
protecting the body from harmful external stimuli as well as aid and
repair the body. However, when dysregulated inflammation can have a
detrimental effect on the body. An immune system that is dysregulated
for an extended period can lead to a variety of autoimmune conditions
such as Multiple Sclerosis, Type 1 Diabetes, Inflammatory Bowel Disease,
or Lupus. (8)
The anti-inflammatory properties of MSCs play a key role in their therapeutic abilities.
How MSCs reduce inflammation
"MSCs from different sources reduce inflammation by decreasing
production of tumor necrosis factor-α (TNF-α) and Interferon-γ (IFN-γ)
and increasing Prostaglandin (PGE2) and Interleukin-6 (IL-6) secretion."
(9)
According to a 2020 study conducted by Gugjoo et al.
"In general, the central role of mesenchymal stem cells (MSCs) in
maintaining homeostasis (immuno-modulation and anti-inflammatory
activities) occurs by interacting with immune cells and is mediated
through cytokines, chemokines, cell surface molecules, and metabolic
pathways. MSCs suppress T-cell proliferation, cytokine secretion, and
cytotoxicity (9)"
MSC secretome and extracellular vesicles (exosome signaling)
The regenerative effects of mesenchymal stem cells do not solely rely on
their differentiation potential and ability to replace the injured
tissues, but also mediated by their secretome via paracrine mechanisms.
(5)
MSC secretome is a set of bioactive factors that are released into the
body including cytokines, growth factors, extracellular vesicles,
neurotrophins, soluble proteins, lipids, and nucleic acids. (5)
The secretomes that are released play important roles in the regulation
of many physiological processes and they are of increasing interest as
potential biomarkers and therapeutic targets in diseases. (10)
According to a 2016 study conducted by Arutyunyan et al. UC-MSCs exhibit
increased secretion of neurotrophic factors such as bFGF, nerve growth
factor (NGF), neurotrophin 3 (NT3), neurotrophin 4 (NT4), and
glial-derived neurotrophic factor (GDNF) compared to bone marrow-derived
(BM-MSCs) and adipose tissue-derived (AT-MSCs). (15)
Additionally, UC-MSCs secrete significantly higher amounts of several
important cytokines and hematopoietic growth factors, including G-CSF,
GM-CSF, LIF, IL-1
α, IL-6, IL-8, and IL-11, compared to BM-MSCs. This suggests that UC-MSCs may be more potent than other sources of MSCs.
Homing properties (how MSCs know where to go)
One of the key benefits of mesenchymal stem cells is their ability to
target specific areas of concern due to their intrinsic homing
capabilities. Mesenchymal stem cell homing, when administered
systemically can be defined as exiting circulation and migrating to the
injury site. (11)
According to a 2019 study conducted by Ullah et al.
Systemic homing is a multistep process governed by specific molecular
interactions. "The process of systemic homing can be split into five
steps: (1) tethering and rolling, (2) activation, (3) arrest, (4)
transmigration or diapedesis, and (5) migration".
This process is outlined in the figure below.
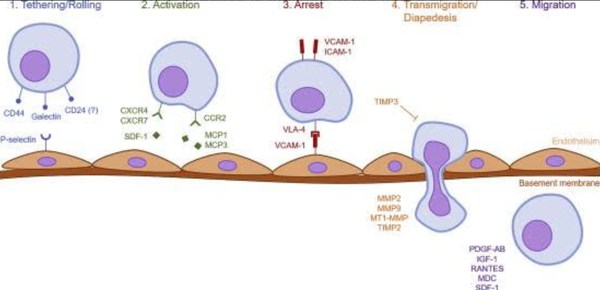
Figure 3. Homing properties of MSCs
Differentiation (becoming new types of cells)
Mesenchymal stem cells are multipotent stem cells that can self-renew
and differentiate into different cell types. In other words, mesenchymal
stem cells can become a variety of different cell types including;
adipose tissue, cartilage, muscle, tendon/ligament, bone, neurons, and
hepatocytes (12)
According to a 2016 study conducted by Almalki et al. - "The
differentiation of MSCs into specific mature cell types is controlled by
various cytokines, growth factors, extracellular matrix molecules, and
transcription factors (TFs). (12)
Mesenchymal stem cells contribute to tissue regeneration and
differentiation, including the maintenance of homeostasis and function,
adaptation to altered metabolic or environmental requirements, and the
repair of damaged tissue. (13)
Conclusion
There is a plethora of research surrounding the mechanisms of
mesenchymal stem cells (MSCs). Many studies have outlined their
diversified capabilities including self-renewal, immunomodulatory,
anti-inflammatory, signaling, and differentiation properties. These
characteristics enable MSCs to be used in a variety of clinical settings
for multiple degenerative conditions.
Research is starting to suggest that umbilical cord tissue-derived MSCs
(UC-MSCs) may be more potent than other sources of mesenchymal stem
cells thus potentially increasing their clinical efficacy. (15)
References:
(1) Torres Crigna, A., Daniele, C., Gamez, C., Medina Balbuena, S., Pastene, D. O., Nardozi, D., … Bieback, K. (2018, June 15).
Stem/Stromal Cells for Treatment of Kidney Injuries With Focus on Preclinical Models. Frontiers in medicine.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6013716/.
(2) Mazini, L., Rochette, L., Amine, M., & Malka, G. (2019, May 22).
Regenerative Capacity of Adipose-Derived Stem Cells (ADSCs), Comparison with Mesenchymal Stem Cells (MSCs). International journal of molecular sciences.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6566837/.
(3) Chu, D.-T., Phuong, T. N. T., Tien, N. L. B., Tran, D. K., Thanh, V. V., Quang, T. L., … Kushekhar, K. (2020, January 21).
An
Update on the Progress of Isolation, Culture, Storage, and Clinical
Application of Human Bone Marrow Mesenchymal Stem/Stromal Cells. International journal of molecular sciences.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7037097/.
(4) Jin, H. J., Bae, Y. K., Kim, M., Kwon, S.-J., Jeon, H. B., Choi, S.
J., Kim, S. W., Yang, Y. S., Oh, W., & Chang, J. W. (2013, September
3).
Comparative analysis of human mesenchymal stem cells from bone
marrow, adipose tissue, and umbilical cord blood as sources of cell
therapy. International journal of molecular sciences.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3794764/.
(5) Liau, L. L., Looi, Q. H., Chia, W. C., Subramaniam, T., Ng, M. H., & Law, J. X. (2020, September 22).
Treatment of spinal cord injury with mesenchymal stem cells. Cell & bioscience.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7510077/.
(6) Jiang, W., & Xu, J. (2020, January).
Immune modulation by mesenchymal stem cells. Cell proliferation.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6985662/.
(7) Bernardo, M. E., & Fibbe, W. E. (2013). Mesenchymal Stromal Cells: Sensors and Switchers of Inflammation.
Cell Stem Cell,
13(4), 392–402.
https://doi.org/10.1016/j.stem.2013.09.006
(8) Ryu, J.-S., Jeong, E.-J., Kim, J.-Y., Park, S. J., Ju, W. S., Kim, C.-H., Kim, J.-S., & Choo, Y.-K. (2020, November 7).
Application of Mesenchymal Stem Cells in Inflammatory and Fibrotic Diseases. International journal of molecular sciences.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7664655/.
(9) Gugjoo, M. B., Hussain, S., Amarpal, Shah, R. A., & Dhama, K. (2020).
Mesenchymal Stem Cell-Mediated Immuno-Modulatory and Anti- Inflammatory Mechanisms in Immune and Allergic Disorders. Recent patents on inflammation & allergy drug discovery.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7509741/.
(10) Stastna, M., & Van Eyk, J. E. (2012, February 1).
Investigating the secretome: lessons about the cells that comprise the heart. Circulation. Cardiovascular genetics.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3282018/.
(11) Ullah, M., Liu, D. D., & Thakor, A. S. (2019, May 31).
Mesenchymal Stromal Cell Homing: Mechanisms and Strategies for Improvement. iScience.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6529790/.
(12) Almalki, S. G., & Agrawal, D. K. (2016).
Key transcription factors in the differentiation of mesenchymal stem cells. Differentiation; research in biological diversity.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5010472/.
(13) Grafe, I., Alexander, S., Peterson, J. R., Snider, T. N., Levi, B., Lee, B., & Mishina, Y. (2018, May 1).
TGF-β Family Signaling in Mesenchymal Differentiation. Cold Spring Harbor perspectives in biology.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5932590/.
(14) Walker, J. T., Keating, A., & Davies, J. E. (2020, May 28).
Stem Cells: Umbilical Cord/Wharton’s Jelly Derived. Cell Engineering and Regeneration.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7992171/.
(15) Arutyunyan, I., Elchaninov, A., Makarov, A., & Fatkhudinov, T. (2016).
Umbilical Cord as Prospective Source for Mesenchymal Stem Cell-Based Therapy. Stem cells international.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5019943/.
Yours in Health and Wellness,
John W Brimhall, DC, BA, BS, FIAMA, DIBAK